Introduction:
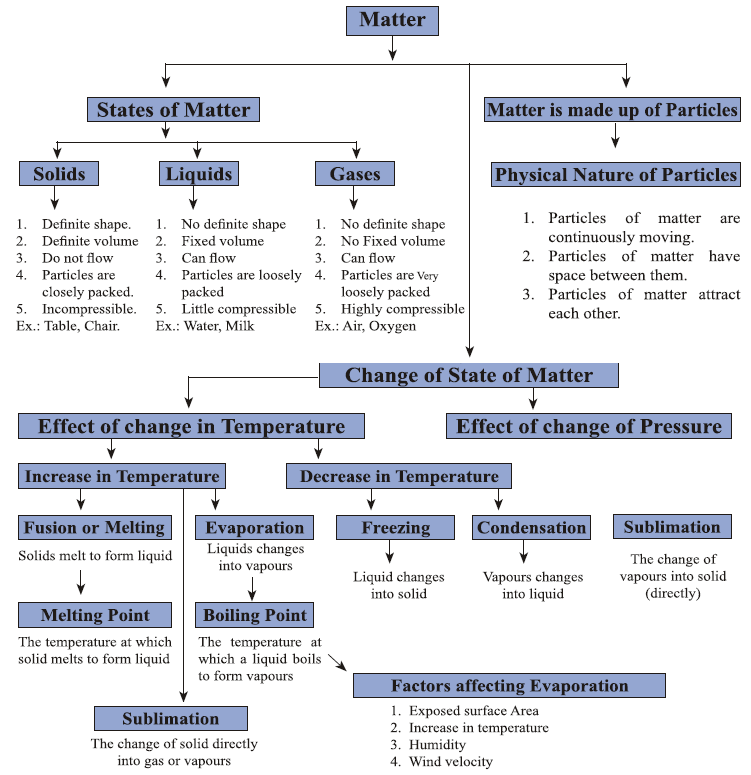
Definition and Characteristics:
●Matter is defined as anything that occupies space, has mass, and offers resistance to any applied force.
●It is the material that composes everything in the universe, including the objects around us and the substances we interact with daily.
●Matter is essential to our understanding of the physical world, as it is the foundation upon which all physical phenomena are based.
Physical Nature of Particles:
● Matter is made up of tiny particles that are too small to be seen with the naked eye.
●These particles are constantly in motion, which means they possess kinetic energy.
● The motion and energy of these particles are influenced by temperature; as the temperature increases, the particles move faster because their kinetic energy increases.
Characteristics of Particles of Matter:
(i) Continuous Movement: Particles of matter are always moving. This continuous motion is due to their inherent kinetic energy, which increases with temperature.
(ii) Space Between Particles: There is space between the particles of matter. This can be observed when substances dissolve in each other. For example, when salt dissolves in water, the salt particles occupy the spaces between the water particles.
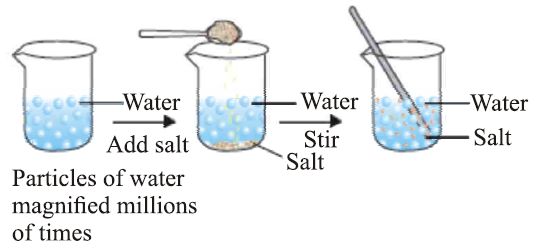
(iii) Attraction Between Particles: Particles of matter attract each other. The strength of this attraction varies depending on the state of matter (solid, liquid, or gas).
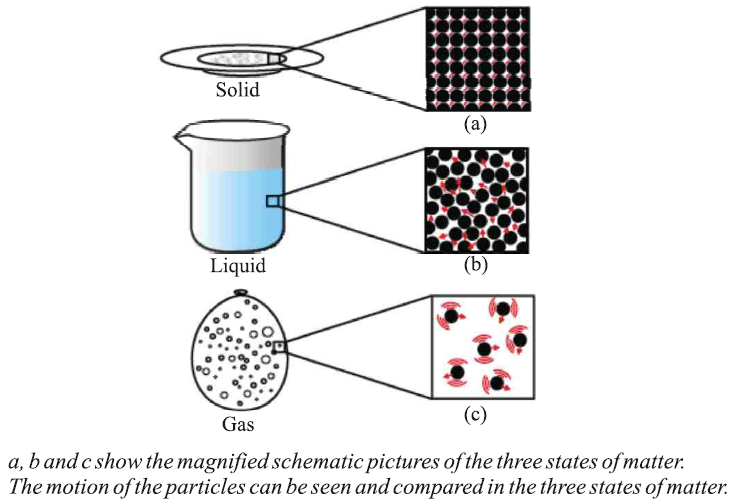
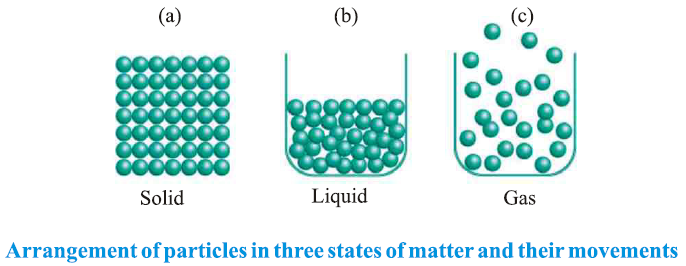
● The space between particles and their kinetic energy are minimal in solids, intermediate in liquids, and maximal in gases.
● The force of attraction between particles is strongest in solids, intermediate in liquids, and weakest in gases.
● Particle movement is minimal in solids, more pronounced in liquids, and maximal in gases
States of Matter:
Matter exists in three primary states: solid, liquid, and gas. Each state has distinct characteristics based on the arrangement and behavior of its particles.
Solid State:
- Characteristics:
- Definite shape and volume
- High rigidity and incompressibility
- Particles are closely packed in a fixed arrangement
- Minimal kinetic energy of particles
- Examples: Bones and teeth in the human body, ice, metals
Liquid State:
- Characteristics:
- Definite volume but no fixed shape
- Particles are less tightly packed than in solids and can move past each other
- Moderate kinetic energy
- Capable of flowing and taking the shape of their container
- Examples: Blood, water, milk
Gaseous State:
- Characteristics:
- No definite shape or volume
- Particles are widely spaced and move freely
- High kinetic energy
- Easily compressible and capable of flowing
- Examples: Oxygen, carbon dioxide, air
Change of State:
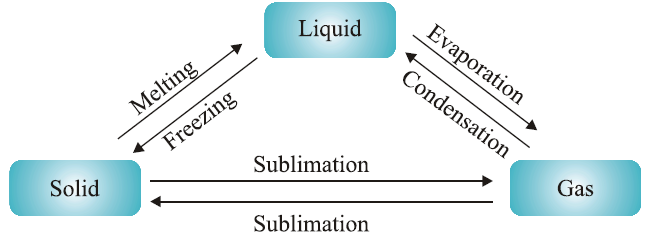
Matter can change from one state to another through the processes of melting, freezing, boiling, condensation, sublimation, and deposition. These changes are influenced by temperature and pressure.
Melting and Freezing:
Melting Point: The temperature at which a solid turns into a liquid. For example, ice melts into water at 0°C (273.16 K).
During melting, the temperature of ice does not rise even though heat is being supplied continuously because of latent heat of fusion. this heat of fusion is used to overcome the forces of attraction between ice particles.
Latent Heat of Fusion: The amount of heat required to change 1 kg of a solid into a liquid at its melting point.
Freezing Point: The temperature at which a liquid turns into a solid. Water freezes into ice at 0°C. The process involves the release of heat as particles lose energy and form a fixed structure.
Boiling and Condensation:
Boiling Point: The temperature at which a liquid turns into vapour. For example, water boils into vapour at 100°C (373 K).
During boiling the temperature of water does not rise even though heat is being supplied continuously as this heat of vapourization is used to overcome the forces of attraction between water particles.
Latent Heat of Vaporization: The amount of heat required to change 1 kg of a liquid into a gas at its boiling point.
Condensation: The process where gas turns into liquid upon cooling. This involves the release of latent heat as gas particles lose energy and come closer to form a liquid.
Sublimation and Deposition:
Sublimation: The direct transition from solid to gas without passing through the liquid state. Examples include dry ice (solid carbon dioxide) and camphor.

Deposition: The direct transition from gas to solid without becoming liquid. This can be seen in frost formation.
Effect of Temperature and Pressure on State Changes
Temperature and pressure are crucial factors that determine the state of matter.
Temperature:
Increase in Temperature: Raises the kinetic energy of particles, causing solids to melt into liquids and liquids to vaporize into gases.
Decrease in Temperature: Lowers the kinetic energy, causing gases to condense into liquids and liquids to freeze into solids.
Pressure:
Increase in Pressure: Can compress gases into liquids or solids by reducing the space between particles.
Decrease in Pressure: Allows particles to move apart, enabling solids or liquids to become gases.
For instance, reducing the pressure on solid carbon dioxide (dry ice) causes it to sublimate directly into carbon dioxide gas without becoming liquid.
Evaporation and Its Effects:
Evaporation is the process where particles on the surface of a liquid gain enough energy to become gas. It is a surface phenomenon that can occur at any temperature below the boiling point.
Factors affect the rate of evaporation:
1. Surface Area: Greater surface area increases evaporation.
2. Temperature: Higher temperatures increase the kinetic energy of particles, leading to faster evaporation.
3. Humidity: Lower humidity levels increase evaporation rates as dry air can absorb more water vapor.
4. Wind Speed: Higher wind speeds remove vapor from the surface quickly, increasing evaporation.
Cooling Effect of Evaporation: When a liquid evaporates, it absorbs heat from its surroundings, leading to a cooling effect. This principle is utilized in various ways, such as in sweating, where the evaporation of sweat cools the body.
Practical Examples:
Acetone on Skin: Applying acetone on the skin leads to a cooling sensation as it evaporates quickly, absorbing heat from the skin.
Wearing Cotton Clothes in Summer: Cotton absorbs sweat and exposes it to the air for evaporation, keeping the body cool.
Sprinkling Water on Ground: Sprinkling water during hot weather helps cool the ground and surrounding air as the water evaporates, taking heat away from the surface.
