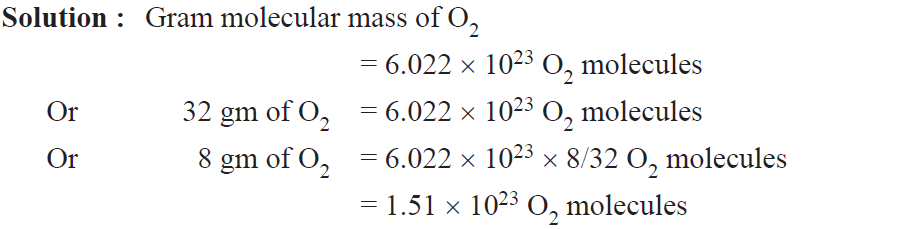Introduction to the Concept of Atoms and Molecules:
Ancient Indian and Greek philosophers thought about the basic nature of matter around 500 BC. Indian philosopher Maharishi Kanad believed that if you kept dividing matter, you would eventually get to tiny particles called “Parmanu.” Similarly, Greek philosophers Democritus and Leucippus came up with the idea of atoms, meaning “indivisible.”
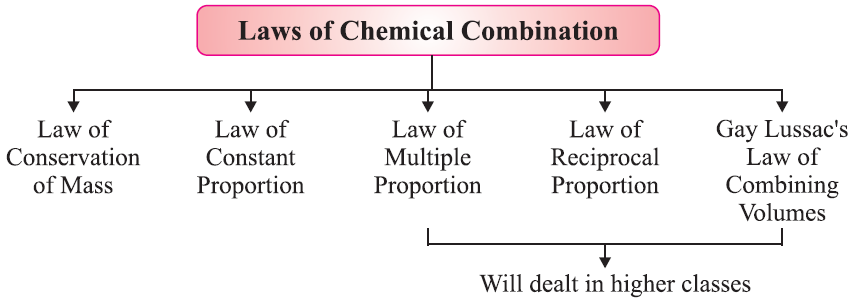
By the late 18th century, scientists understood the difference between elements and compounds. This led to questions about how elements combine. Antoine Lavoisier and Joseph Proust created the basic rules for modern chemistry with their laws of chemical combination.
Law of Conservation of Mass:
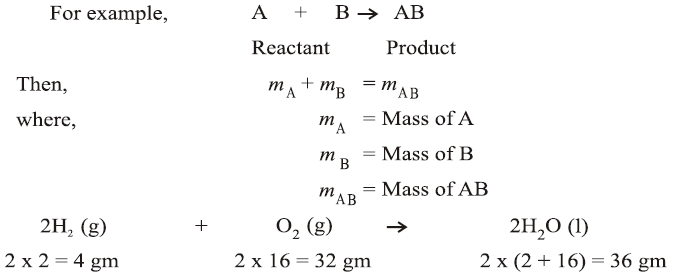
This law states that mass can neither be created nor destroyed in a chemical reaction. For example, when a chemical reaction occurs in a closed system, the total mass of the reactants equals the total mass of the products.
Example : In a reaction 5.3 gm of sodium carbonate reacted with 6 gm of ethanoic acid. The products were 2.2 gm of CO2, 0.9 gm of H2O and 8.2 gm of sodium ethanoate. Show that these observation are all in agreement with law of conservation of mass.
Solution:
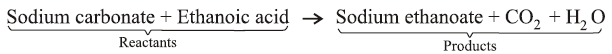
Now, according to the law of conservation of mass :
Mass of sodium carbonate + Mass of ethanoic acid = Mass of sodium ethanoate + Mass of CO2 + Mass of H2O
Putting values of masses from the equation :
5.3 gm + 6.0 gm = 8.2 gm + 2.2 gm + 0.9 gm
Or 11.3 gm = 11.3 gm
Since, LHS = RHS
∴ Law of conservation of mass is in agreement with the given values in equation.
Law of Constant Proportion:
This law states that a chemical compound always contains the same elements in the same proportion by mass, regardless of its source or method of preparation. For instance, water (H₂O) always contains hydrogen and oxygen in a mass ratio of 1:8.
For example :
18 gm of H2O ⇒ 16 gm of oxygen + 2 gm of hydrogen,
i.e., mH/mO = 2/16 = 1/8
36 gm of H2O ⇒ 32 gm of oxygen + 4 gm of hydrogen,
i.e., mH/mO = 4/32 = 1/8
09 gm of H2O ⇒ 08 gm of oxygen + 1 gm of hydrogen,
i.e., mH/mO = 1/8
From the above three cases, differently weighing H2O samples were taken but the ratio of masses of ‘H’ to mass of ‘O’ comes out to be ‘1/8’ is same, proving law of constant proportion.
Likewise, if a sample of ‘H2O’ was taken from anywhere i.e., from well, pond, lake or anywhere the ratio of masses of ‘H’ to ‘O’ will come out to be same as ‘1/8’.
Example : Hydrogen and oxygen combine in the ratio 1 : 8 by mass to form water. What mass of oxygen gas would be required to react completely with 3.0 gm of hydrogen gas ?

Dalton’s Atomic Theory:
Based upon laws of chemical combination, Dalton’s Atomic Theory provided an explanation for the Law of Conservation of Mass and Law of Constant Composition.
Postulates of Dalton’s atomic theory are as follows :
(i) All matter is made up of very tiny particles called ‘Atoms’.
(ii) Atom are indivisible particles, which can’t be created or destroyed in a
chemical reaction. (Proves ‘Law of Conservation of Mass’)
(iii) Atoms of an element have identical mass and chemical properties.
(iv) Atoms of different elements have different mass and chemical properties.
(v) Atom combine in the ratio of small whole numbers to form compounds.
(Proves ‘Law of Constant Proportion’)
(vi) The relative number and kinds of atoms are constant in a given compound.
What is Atom?
According to modern atomic theory, an atom is the smallest particle ofan element which takes part in chemical reaction such that during the chemical reaction, the atom maintain its identity, throughout the chemical or physical change.
●Atoms are very small and hence can’t be seen even through very powerful microscope.
●Atomic radius of smallest atom in hydrogen is 0.37 x 10-9 or 0.037 nm. Such that, 1nm = 10-9 m
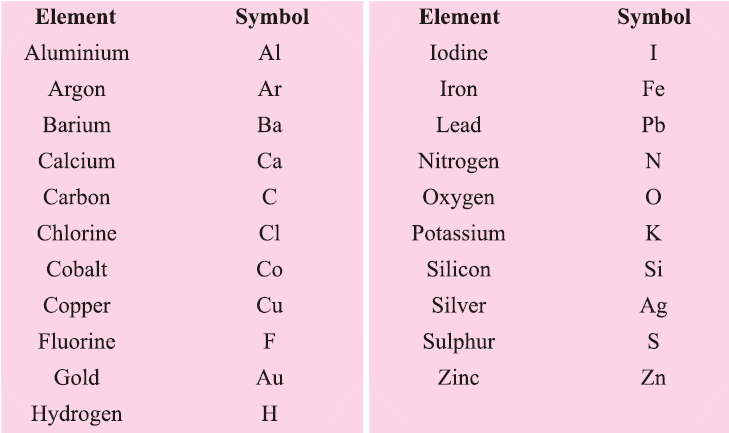
IUPAC (International Union of Pure & Applied Chemistry) Symbols of Atoms of Different Elements
Atomic Mass
●The mass of an atom of an element is called its atomic mass.
●In 1961, IUPAC have accepted ‘atomic mass unit’ (u) to express atomic and molecular mass of elements and compounds.
Atomic Mass Unit
The atomic mass unit is defined as the quantity of mass equal to 1/12 of mass of an atom of carbon-12.
1 amu or u = 1/12 x Mass of an atom of C12
1 u = 1.66 x 10-27 kg
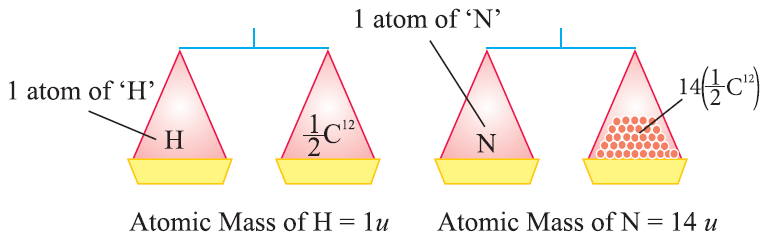
Likewise,
| Element | Atomic Mass |
| Hydrogen | 1 u |
| Carbon | 12 u |
| Nitrogen | 14 u |
| Oxygen | 16 u |
| Sodium | 23 u |
| Magnesium | 24 u |
| Sulphur | 32 u |
| Chlorine | 35.5 u |
| Calcium | 40 u |
How do atoms exist ?
●Atoms of most of the elements are very reactive and does not exist in free state.
●Only the atoms of noble gases (such as He, Ne, Ar, Kr, Xe and Rn) are chemically unreactive and can exist in the free state as single atom.
●Atoms of all other elements combine together to form molecules or ions.
What is Molecule?
●A molecule is a group of two or more atoms which are chemically bonded with each other.
●A molecule is the smallest particle of matter (except element) which is capable of an independent existence and show all properties of that substance. E.g., ‘H2O’ is the smallest particle of water which shows all the properties of water.
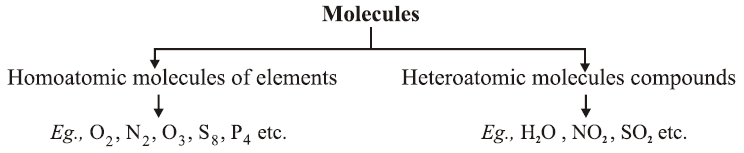
●A molecule may have atom of same or different elements, depending upon this, molecule can be categorized into two categories :
●Homoatomic molecules (containing atom of same element) and Heteroatomic molecules or compounds (containing atoms of different elements).
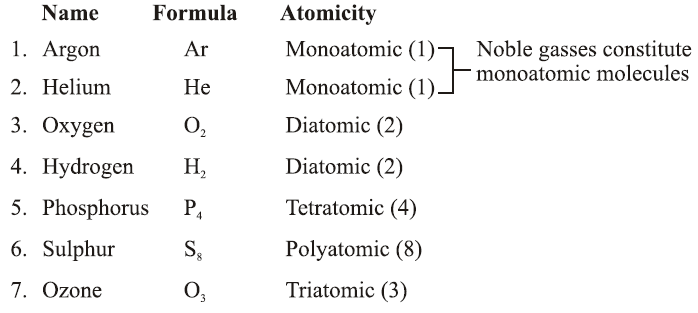
Atomicity:
The number of atoms present in one molecule of an element is called its atomicity. Name Formula Atomicity
Chemical formulae:
It is the symbolic representation of the composition of a compound.
Characteristics of chemical formulae:
●The valencies or charges on ion must balance.
●When a compound is formed of metal and non-metal, symbol of metal comes first. E.g., CaO, NaCl, CuO.
●When polyatomic ions are used, the ions are enclosed in brackets before writing the number to show the ratio. E.g., Ca(OH)2, (NH4)2SO4
Molecular Mass:
It is the sum of atomic masses of all the atoms in a molecule of that substance.
E.g., Molecular mass of H2O = 2 x Atomic mass of Hydrogen + 1 x Atomic mass of Oxygen
So, Molecular mass of H2O = 2 x 1 + 1 x 16 = 18 u
Formula Unit Mass:
It is the sum of atomic mass of ions and atoms present in formula for a compound.
E.g., In NaCl, Na = 23 a.m.u. Cl = 35.5 a.m.u.
So, Formula unit mass = 1 x 23 + 1 x 35.5 = 58.5 u
Rules for writing chemical formulae
(i) We first write symbols of elements which form compound.
(ii) Below the symbol of each element, we should write their valency.
(iii) Now cross over the valencies of combining atoms.
(iv) With first atom, we write the valency of second atom (as a subscript).
(v) With second atom, we write the valency of first atom (subscript).
Examples :
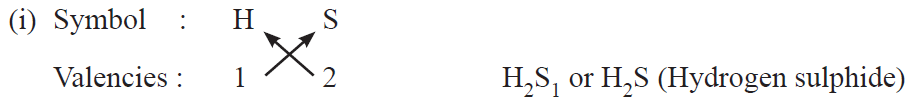
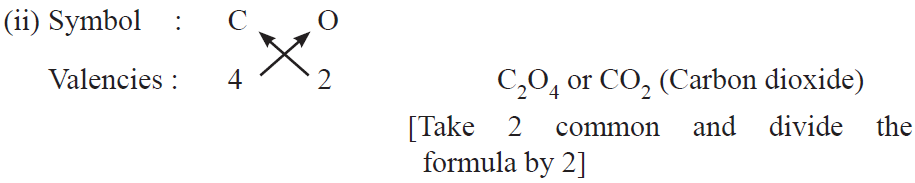
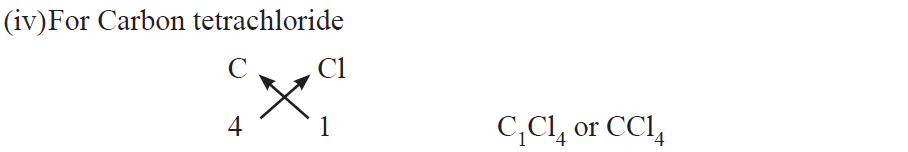
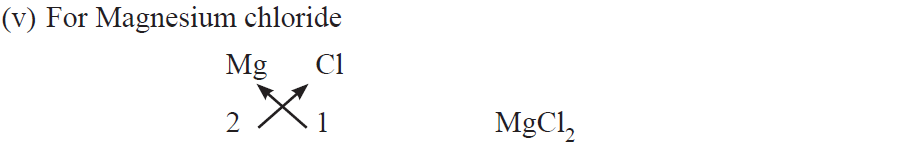

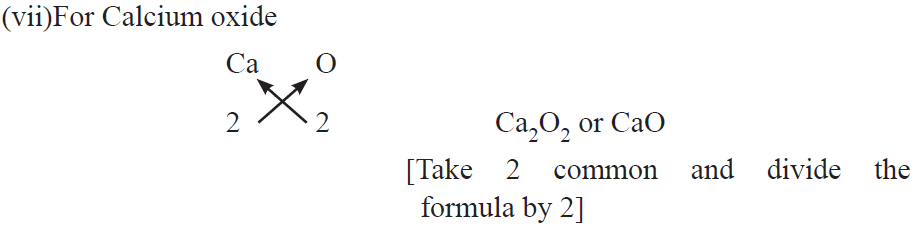
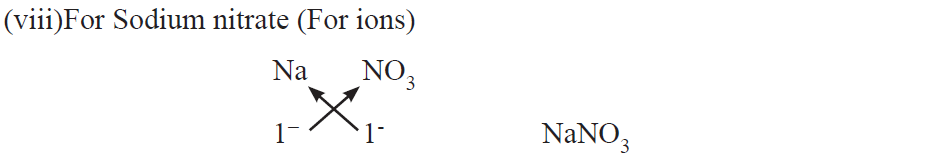
Ions:
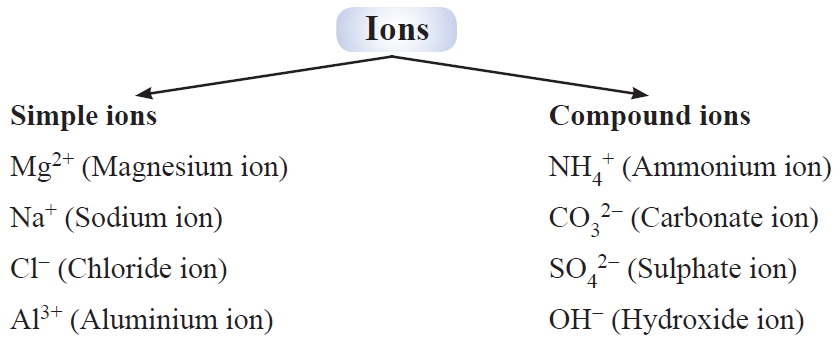
An ion may be defined as an atom or group of atoms having positive or negative charge.
Some positively charged ions : Na+ , K+, Ca2+, Al3+
Some negatively charged ions : Cl– (chloride ion), S2– (sulphide ion), OH– (hydroxide ion), SO4 2- (sulphate ion)
Chemical formulae of Ionic Compounds (Polyatomic)
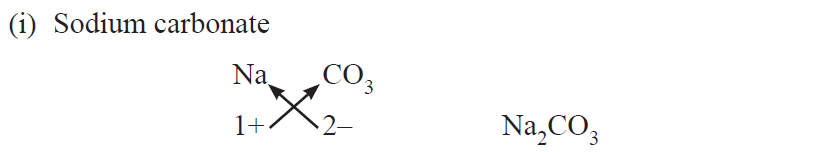
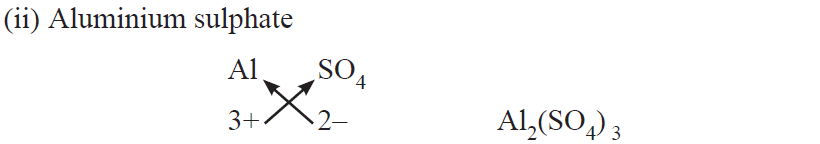
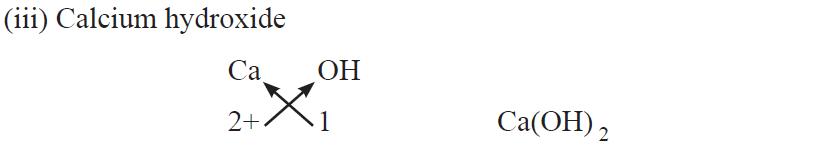
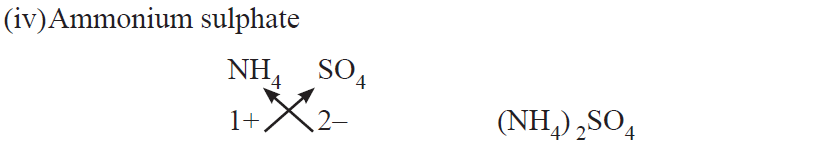
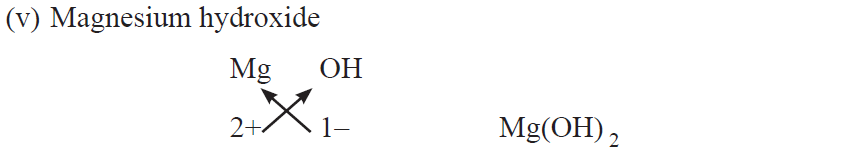
Molar Mass:
The molar mass of a substance is the mass of 1 mole of that substance. It is equal to the 6.022 x 1023 atoms of element/ substsnce.
Example :
(a) Atomic mass of hydrogen (H) is 1 u. Its molar mass is 1 g/mol.
(b) Atomic mass of nitrogen is 14 u. So, molar mass of nitrogen (N) is 14 g/mol.
(c) Molar mass of S8 = Mass of S 8 = 32 x 8 = 256 g/mol
(d) Molar mass of HCl = Mass of H + Mass of Cl = 1 + 35.5 = 36.5 g/mol
Mole concept:
A group of 6.022 x 1023 particles (atoms, molecules or ions) of a substance.
1 mole of atoms = 6.022 x 1023 atoms
1 mole of molecules = 6.022 x 1023 molecules
Example, 1 mole of oxygen = 6.022 x 1023 oxygen atoms
•6.022 x 1023 is Avogadro Number (L).
•1 mole of atoms of an element has a mass equal to gram atomic mass of the element.
Important Formulae:
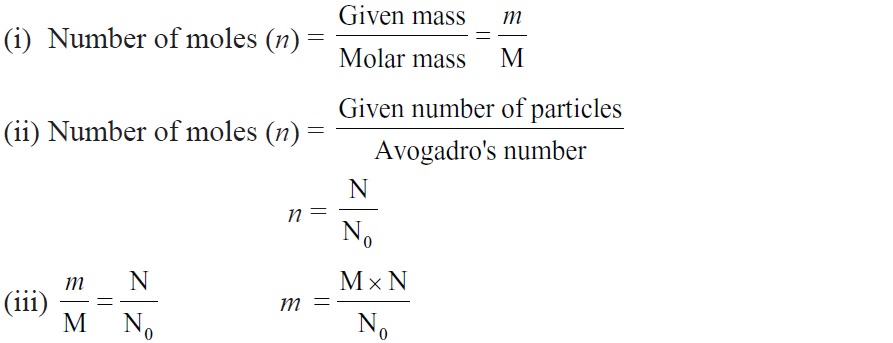
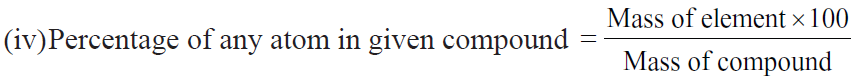
Example. Calculate no. of iron atoms in a piece of iron weighing 2.8 gm (At. mass = 54 u).
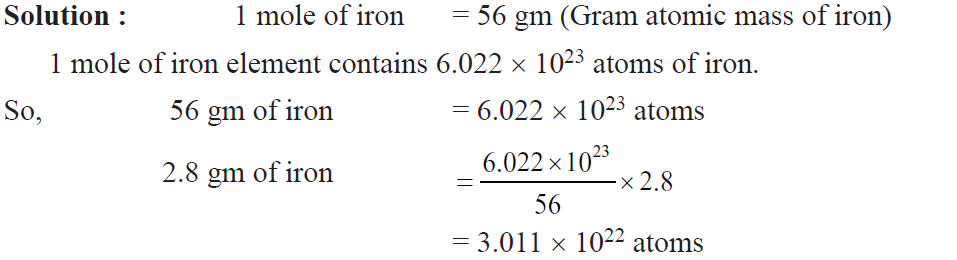
Example. Mass of one molecule of a substance is 5.32 x 10-23 g. What is its molecular mass ?
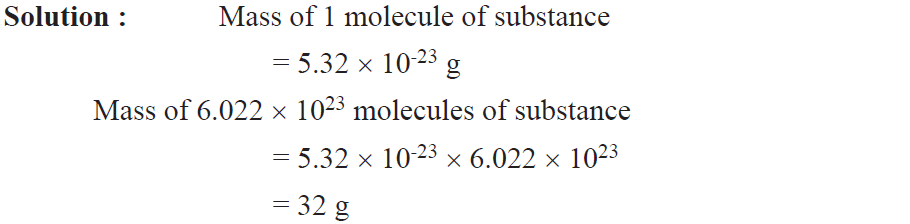
Example. Calculate the mass of 0.5 mole of N2 gas.
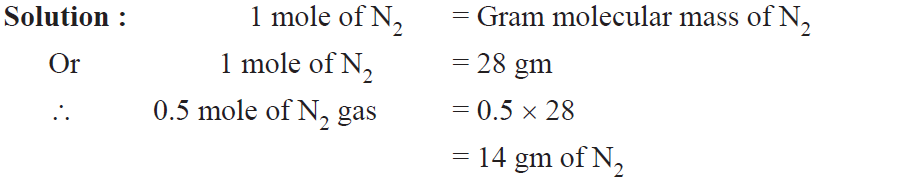
Example. Calculate the total number of O2 molecules present in 8 gm of O2.